Is it Likely that Hormonal Contraceptive Induced Desynchronization Will Be Overcome During Pregnancy?
The Case for Embryo-Endometrial Synchrony
It is widely accepted in the research community that a successful pregnancy and birth requires embryo-endometrial (E/E) synchrony. Lack of progress improving in-vitro fertilization (IVF) outcomes reinforces that pregnancy is a finely-tuned process that is difficult to re-create successfully in the lab.
“Despite the increasing use of IVF and related assisted reproductive technologies worldwide, success rates are still modest, with only around 25–30% of cycles resulting in a live birth (de Mouzon et al., 2020)”1 (McMahon et al., 2024)
The nature of intricate and interrelated events occurring throughout pregnancy could well be described as a symphony orchestra in synchrony where musicians playing out-of-tune or out-of-time could have disastrous effects.
Figure 1 (Figure 3 from the source) is from an article titled, “What is the Contribution of Embryo-endometrial Asynchrony to Implantation Failure?” by Teh, McBain, and Rogers in 2016.2 It illustrates the asynchronization common in controlled ovarian hyperstimulation protocols used during IVF that leads to implantation failure.
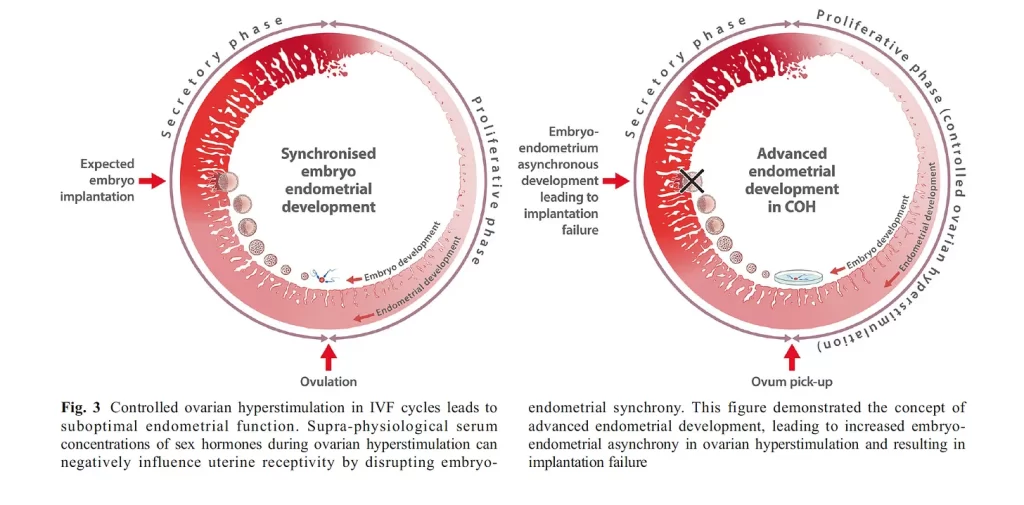
Figure 1 above depicts a normally growing endometrium that is mis-timed with the fetal embryo transfer. If timing is off by more than +/- 1.5 days of synchrony, success rates are reduced.
In a similar manner, hormonal contraceptive (HC) use leaves a woman with a desynchronized system in the event of pregnancy but is further confounded by an underdeveloped endometrium.

Figure 2. Modified image from Teh, McBain, and Rogers, 2016. Desynchronization combined with inadequate endometrial development in the event of pregnancy during use of hormonal contraceptives.
Synchronization requires not only proper timing of events, but also proper preparation and functioning of processes. Poor timing and disruption of fetal-maternal communication increases risk of pregnancy loss.
Early pregnancy loss was once considered a “black box” of knowledge3 (Macklon, 2002). But we are learning more and more:
“Once considered a black box, our understanding of the mechanisms that control human embryo implantation is accelerating rapidly, aided by powerful new technologies including single-cell ‘omics’, spatial transcriptomics, blastoids, organoids and assembloids.”4 (Muter, 2023)
While we might not yet know all the reasons pregnancies fail, it is universally acknowledged that a successful, normal live-birth outcome requires near perfect conditions. By “near perfect” I mean a high level of embryo-endometrial synchrony which requires normal hormone levels and timing throughout the entire pregnancy process. This includes the proliferative phase (pre-fertilization) and the luteal phase (post-fertilization).
Consider the following research from the fields of endocrinology, infertility, and IVF, which emphasize carefully orchestrated synchronization and delicate communication signaling processes involved in menstruation and pregnancy:
“Ovulation follows a carefully orchestrated series of neuroendocrine and intrafollicular events.”5 (Devoto, Kohen, Munoz, Struass, 2009, S19)
“The synchronized development of a viable embryo and a receptive endometrium is critical for successful implantation to take place.” (Teh, McBain, Rogers, 2016, p. 1419)
“To produce a viable normal embryo, a sequence of perfectly linked processes must occur in the post-menarchal ovary.”6 (Devesa, Caicedo, 2019, p. 6)
“The formation of a functional corpus luteum relies on the appropriate proliferation and differentiation of both granulosa and theca cells. Any disruption in the crosstalk between granulosa and theca cell or differentiation of these cells types alters lineages and gene expression profiles that could negatively impact luteinization and progesterone production.”7 (Abedel-Majed, Romereim, Davis , Cupp, 2019, p. 11)
“A specific hormone signaling sequence is involved in each phase and stimulates the endometrium to first proliferate and then transform to a receptive state.”8 (Gao et al., 2019, p. 546)
“…the mechanisms driving granulosa cell (GC) progesterone production are much more intricate than previously recognized, with both local and systemic factors interacting to promote successful GC function and physiological fine-tuning of progesterone levels.”9 (DeWitt, Whirledge, Kallen, 2020, p. 2)
“A coordinated sequence of events must occur in order to establish and successfully maintain a healthy pregnancy. Synchrony between the development of the early embryo and establishment of a receptive endometrium is necessary to allow implantation and subsequent progression of pregnancy.”10 (Tal, Hugh, Taylor, 2021)
“LH and FSH production and activity depend on the precise orchestration of numerous elements of the hypothalamic–pituitary–gonadal (HPG) axis…”11 (Bosch et al., 2021, p. 1470)
“Before successful implantation of a blastocyst in the pre-decidualized endometrium, morphological and biochemical changes of both the embryo and the endometrium must be in synchrony.”12 (Bulletti, Bulletti, Sciorio, Guido, 2022, p. 1)
“While embryo quality is a central determinant of implantation and pregnancy success, temporally coordinated differentiation of endometrial stromal cells into DSCs to attain uterine receptivity, and a synchronized cross-talk between maternal and embryonic tissues are crucial for successful implantation”13 (Abuwala, Tal, 2021, p. 6)
“While embryo development and endometrial preparation are concurrent yet independent processes, their synchronization is critical to the success of embryo apposition, adhesion, invasion, and further ongoing pregnancy….When synchrony is lost or receptivity is not achieved, the consequence is early pregnancy loss or infertility….Embryo implantation requires a receptive endometrium, a functional, normally developing embryo, and synchronized embryo-endometrial cross-talk.”14 (Blanco-Breindel, Singh, Kahn, 2023)
“The process of implantation involves a complex sequence of cellular steps that must progress correctly for pregnancy to occur.”15 (Hull, Robertson, 2023)
“Successful implantation requires the exquisitely coordinated migration and invasion of trophoblast cells from the outer capsule of the blastocyst into the endometrium.”16 (Benagiano,Mancuso, Guo, Renzo, 2023, p. 8)
“The endometrium undergoes a complex series of changes during the menstrual cycle in preparation for embryo implantation. This process requires precise coordination between the embryo and the endometrium [8]. Any disruption can lead to RPL or RIF”17 (Braun et al., 2023, p. 2)
“It has been discovered that the complex and delicate dialogs between trophoblasts and DICs [decidual immune cells] are the key link in driving the establishment and maintenance of maternal–fetal immunotolerance.”18 (Yao, Ye, Chen, Zhang, Cai, & Zheng, 2024, p. 3) [addition mine]
In summary, according to the research, a healthy birth requires:
- synchronized embryo-endometrial crosstalk
- sequence of perfectly linked processes
- no disruption in embryo-endometrial crosstalk
- specific hormone signal sequencing
- fine tuning of hormone levels
- intricate, finely-tuned mechanisms
- precise orchestration
- complex and delicate dialogues
- complex sequence of cellular steps that must progress correctly
Protestant hedged contraceptionists have faith that in the event a woman gets pregnant while on HC, a system that has been chronically broken and desynchronized for months or years can be rejuvenated and re-synchronized in about 6-10 days (the time from fertilization to implantation) while the woman is presumably still taking HC since pregnancy recognition may be difficult at such an early stage.
One aspect of embryo-endometrial synchrony is the proper development of the follicle prior to ovulation and the synchronization of egg release, embryo development, and endometrial preparation.
This means that synchronization in pregnancy is not an entirely linear, event-driven phenomenon. Concurrent cyclic processes are happening throughout the body for the duration of the menstrual cycle.
Historically, political questions surrounding the effects of HC have been somewhat linear— before or after fertilization, before or after implantation—as if new and independent circumstances are presented in each phase.
But the phases are interconnected and synchronized from the start. For instance, HC seems to be affecting follicle development prior to ovulation:
“The balance of the evidence is that the manipulation of ovarian activity by hormonal contraceptives may not only act on the ovulation process, but may also influence the very early stages of folliculogenesis, as demonstrated by the changes in AMH concentration and AFC during contraceptive use.” (LaMarca et al., 2023, p. 7)
Follicle development can affect oocyte maturation. Oocyte maturation can effect embryo-maternal signaling pathways with the endometrium. Miscommunication with and malpreparation of the endometrium can affect implantation.
Even if an endometrium was “somewhat” prepared despite HC use (which research suggests would be difficult), problems—induced by HC— with the developing follicle during folliculogenesis could lead an embryo to be rejected by the endometrium. Some would call this a “post-fertilization effect.” But it was the effects on the follicle during development—or pre-fertilization—that led to the implantation failure.
This is why I use the term “contraceptive related induced proliferative and luteal effects” (CRIPLE). This term encompasses the effects of HC throughout the entire cycle of menstruation and/or pregnancy until the placenta rescues the corpus luteum. The proliferative phase generally encompasses the time from the end of menstruation to ovulation. The luteal phase generally covers the time from ovulation to implantation.
In light of what we now know about the synchronized and interconnected processes that are occurring, it is outdated, oversimplified, and insufficient to continue using the old paradigm of pre and post-fertilization effects.
The Desynchronizing Effects of HC
The pregnancy process was never meant to be broken. But hormonal contraception (HC) breaks it well. The primary mechanism of action of all hormonal contraception is manipulation of the hypothalamic-pituitry-gonadal (HPG) axis to create an abnormal hormonal state (specifically relating to estrogen and progesterone).
What are the effects of this type of hormone imbalance?
Two effects that are frequently emphasized are reduced ovulation and thickening of cervical mucus. These are emphasized because they are true contraceptives when they prevent pregnancy.
But what about endometrial effects?
In the past, denial of these effects was more common. But these effects are widely acknowledged in the infertility and artificial reproductive technology (ART) research communities.
The following sources are from 2018 and later.
One mechanism of progestogens is “desynchronization of the endometrial changes necessary for implantation.”19 (Regidor, 2018, p. 1)
“Increasing evidence suggests that impaired decidualization predisposes to late implantation, causes quality control malfunction of embryo development, and induces early placental insufficiency, regardless of the embryonic karyotype; thus, recurrent pregnancy loss is likely to be the result of these processes.”20 (Murata, 2021, p. 3)
“Progestins may prevent implantation because of their atrophic effect on the endometrium.”21 (Nagy, 2021, p. 8)
“Our studies indicated that thin endometrium not only had detrimental effect on pregnancy outcomes, but also increased the risk of HDP (hypertensive disorders in pregnancy) in women and SGA (small gestational age) of babies, or decreased BW of babies.”22 (Liao, Liu, Cai, Shen, Sui, Zhang, Qian, 2022, p. 2)
“…when the progestin-only pill is administered, the constant low level of a progestogen leads to the development of endometrial thinning with scanty and atrophied glands and very much reduced synthesis of progesterone receptors, which is hostile towards the implantation of a fertilized ovum. The exposure of the endometrium to progestogen without prior priming by estrogen, in the case of the POP, contributes to endometrial instability.”23 (Liji, 2022)
“Progestins prevent pregnancy by suppressing ovulation, thickening cervical mucus, and causing the endometrium to become atrophic.”24 (Yland et al., 2023, p. 9)
“It is well recognized that endometrial thinning occurs during the use of combined oral contraceptive (COC) and hormonal long-acting reversible contraceptives, such as the levonorgestrel intra-uterine device (IUD) or depot medroxyprogesteronacetate (DMPA). After prolonged use, endometrial atrophy develops (Anderson et al., 2005; Grow and Iromloo, 2006; Dinehart et al., 2020). Possibly, the thinning effect of hormonal contraceptives on the endometrium lingers longer than previously anticipated.”25 (Homminga, Meer, Groen, Cantineau, Hoek, 2023, p. 238)
“Insufficient endometrial growth prior to ovulation, absence of endometrial compaction following ovulation, and lack of peri-implantation junctional zone remodelling are all associated clinically with increased risk of implantation failure.” (Muter, 2023, p. 6)
In one animal study, horses were given cloprostenol to to reduce their progesterone levels on days 0–3 after ovulation to induce subphysiological progesterone concentrations. 4 of 7 pregnancies in that group were lost or compromised compared to 100% healthy births in the control group (Wagner et al., 2023). The authors concluded:
“…reduced progesterone concentration in the early luteal phase leads to delayed conceptus growth beyond placentation and increased pregnancy loss.”26 (Wagner et al., 2023, p. 1)
In summary, hormone imbalance and embryo-endometrial desynchronization—the environment created by using HC—leads to an increased risk of:
- hypertensive disorders
- reduced synthesis of progesterone receptors
- malfunction of embryo development
- desynchronization of the endometrial changes necessary for implantation
- insufficient endometrial growth prior to ovulation
- absence of endometrial compaction following ovulation
- lack of peri-implantation junctional zone remodelling
- impaired decidualization
- atrophic endometrium
- endometrial instability
- late implantation
- placental insufficiency
- small gestational age
- decreased birth weight
- recurrent pregnancy loss
Is It Likely that Adequate Re-Synchronization Can Occur on HC?
Protestant hedged contraceptionists (see “The Protestant Pill Problem”) must have faith that in the event of pregnancy while on HC, a system that has been atrophied and desynchronized for months or years can be rejuvenated and re-synchronized to an adequate state in the short time from fertilization to implantation.
What do the studies say about how long it takes to get back to normal after discontinuing HC?
One to two months according to the research. Additionally, research studies requiring women with a normal cycle generally require that they have not used HC within the last 1-2 months.
“After discontinuation of long-term use of COC, AMH concentration increased by 53% and AFC increased by 41%, with values returning to normal within 2 months.”27 (Lamarca et al., 2023, p. 7)
“Menstrual cycle biomarkers are altered for at least two cycles after discontinuation of OCs, and this may help explain the temporary decrease in fecundity associated with recent OC use.”28 (Nassaralla et al., 2011)
A delay in fertility is expected when a woman stops using HC.
Reading Between the Lines
A study in 2023 involved women who stopped using HC and were trying to get pregnant. It included 13,460 women aged 21-45 years who were planning a pregnancy, and 8,899 conceived over a 9 year period. (Yland, 2023)
The study is about miscarriage rates, but what I’m reading between the lines is how long it took the women to get pregnant after recent HC use, and that data is found in Table 1 in the row “total number of menstrual cycles tried to conceive.”
For this example, I’ll use data from the oral contraception group because they had the highest pregnancy rate and one of the lowest miscarriage rates in the study. (Other HC methods had less pregnancies and more miscarriages; injectables were the worst.)
For the 2,506 women who conceived where oral contraception was the most recent method of contraception used, the mean number of menstrual cycles tried to conceive was 6.8 with a standard deviation of 5.7. (Yland, 2023, Table 1)
I asked ChatGPT to make me a chart:
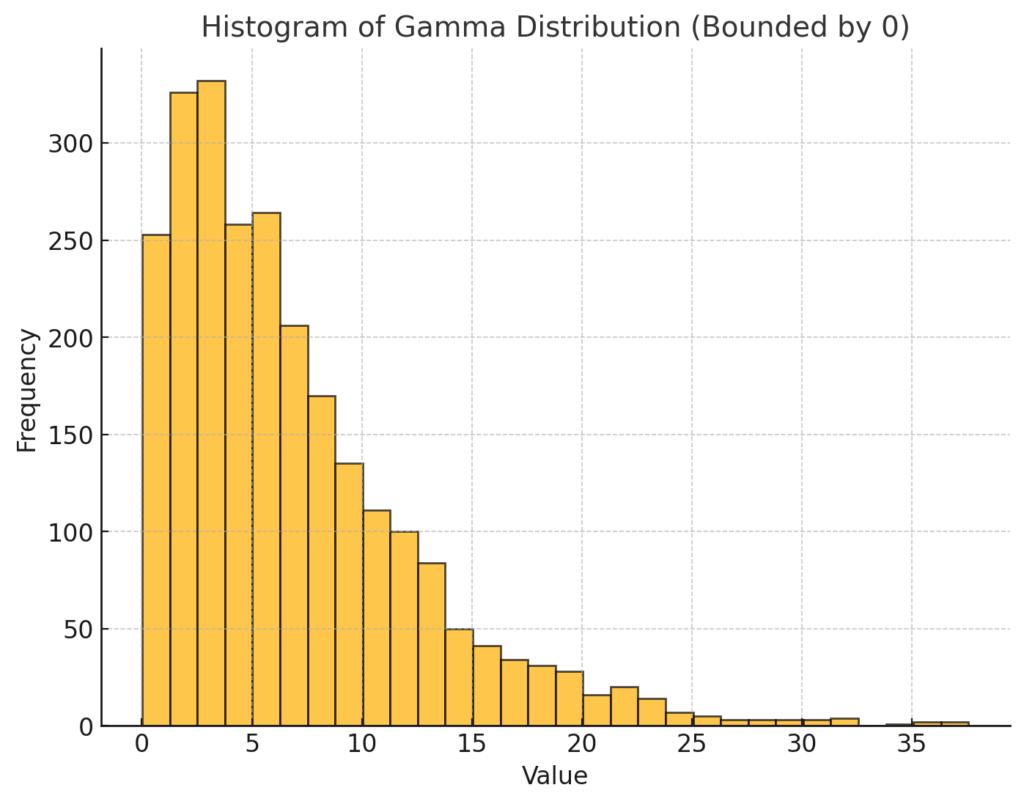
Figure 3. ChatGPT derived chart using n=2506, mean=6.8, std dev=5.7. The chart reflects data from Yland, 2023, Table 1, under the row “total number of menstrual cycles tried to conceive” for the column labeled “Oral Contraceptive.” The x-axis (Value) represents number of menstrual cycles tried and the y-axis (Frequency) represents number of pregnancies
The x-axis (Value) in Figure 3 represents number of menstrual cycles tried and the y-axis (Frequency) represents number of pregnancies (n=2506, mean=6.8, std dev=5.7)
I then asked ChatGPT to give me the percentage of the whole for each value, using whole numbers. It gave me the data which I downloaded into excel. I then highlighted the cumulative pregnancy rates for 1, 3, 6, and 12 cycles (months).
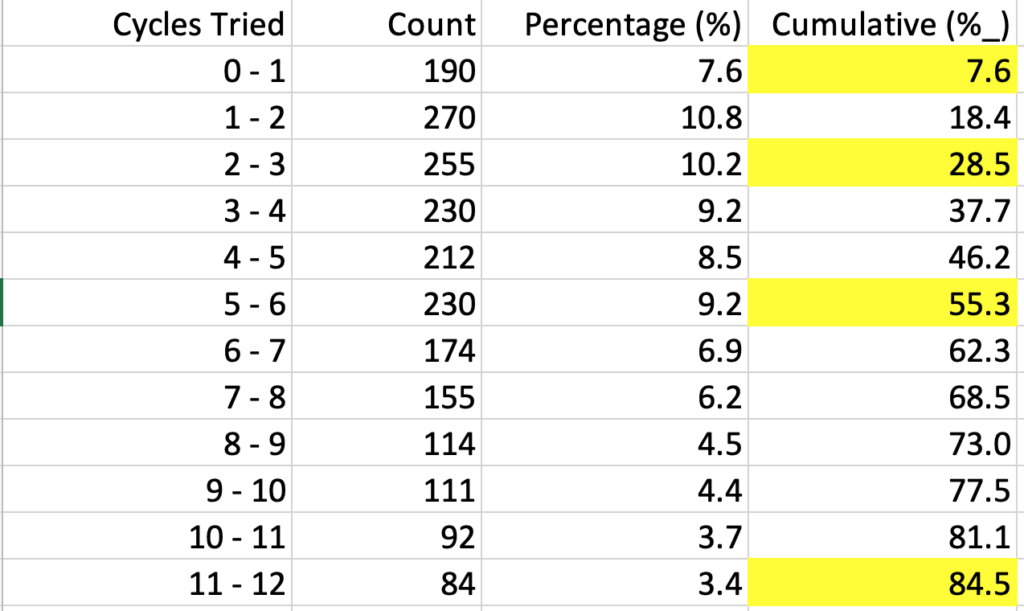
Figure 4. ChatGPT derived data from Figure 3 for cycles 1-12 (abridged). Cumulative pregnancy rates for 1, 3, 6, and 12 months are highlighted.
As seen in Figure 4, the cumulative pregnancy rates for 1, 3, 6, and 12 months after recent use of HC was 8%, 29%, 55%, and 85%. (This is not raw data, but an estimate based on the mean and standard deviation from Figure 3.)
How does this compare to couples trying to get pregnant with less previous HC use?
A study by Gnoth, Godehardt, Godehardt, Frank-Herrmann, and Freundl in 2003 had a sample size of 346 women who were trying to get pregnant. Only 20% reported previous use of oral contraception, the rest were using natural family planning. All participants used the symptom-thermal method and timed intercourse in their attempts to get pregnant.
Cumulative pregnancy rates at 1, 3, 6, and 12 months was 38%, 68%, 81% and 92% respectively).29 (Gnoth, 2003)
- In the Gnoth study (20% previous OC use), 68% of women were pregnant after 3 months. (hard data)
- In the Yland study (100% previous OC use), 29% of women were pregnant after 3 months. (estimated)
Even if you discount my estimated numbers from the Yland data, it is widely recognized that HC use causes delayed fertility after discontinuation. In other words, it is likely you are infertile for at least 1-2 months after stopping the use of hormonal contraception. The system clearly needs time to recover.
That might be a minor delay for couples trying to conceive.
But a 30-60-day system recovery time is a major challenge to Protestant hedged contraceptionists suggesting it can recover in 6-10 days.
In Corpus Luteum We Trust?
C. Ryan Fields in his article “The Christian and Oral Contraceptives: An Investigation into Moral Permissibility” says, “there is increasing reason to question the legitimacy of the theory that the reduction of uterine lining associated with oral contraceptive use actually harms the fertilized egg” (Fields, 2020). He cites a suggestion by Sullivan that the formation of a corpus luteum might be enough to get everything back on track for a normal healthy birth.
Fields (2020) quoting Sullivan (2006):
“…’if [breakthrough] ovulation takes place, a completely different hormonal milieu comes into existence [because] ovulation leaves behind the corpus luteum, a rich source of estrogen and progesterone. After the six days required for the embryo to travel down the uterine tube into the uterus, these hormones [would] have transformed the endometrium, [making it] receptive for implantation.’ In short, Sullivan argues that we have good reason to think that the ‘hostile endometrium’ pointed to by the pill’s opponents is, by the time of implantation, actually transformed into a sufficiently ‘hospitable’ one.”30 (Fields, 2020)
Sullivan is putting forth an accelerated re-synchronization theory and Fields seems open to this.
But what makes Sullivan think that a corpus luteum formed in suboptimal conditions will perform adequately? He provides no data to support this theory.
Quite the opposite, in “The Inadequate Corpus Luteum,” (2021) W. Colin Duncan discusses several scenarios that would result in an inadequate corpus luteum, including:
- follicle with suboptimal growth
- inadequate or premature LH surge
“There is good evidence that a suboptimal LH surge would be associated with an inadequate corpus luteum.”31 (Duncan, 2021)
“Poor follicular growth is also associated with a reduction in oocyte quality. A follicle with suboptimal growth, or one seeing a premature LH surge, is not a suitable environment for normal oocyte development and would not form a normally functioning corpus luteum.” (Duncan, 2021)
These conditions are common in women with PCOS and for those undergoing controlled ovarian hyper stimulation during IVF. These conditions are also created by hormonal contraception when a corpus luteum forms.
Duncan explains, “If a CL is not producing enough progesterone it usually means there is a problem with the growing or releasing of the egg.” (Duncan, 2021)
This is directly related to follicule-stimulating hormone (FSH) production and effectiveness during the follicular phase.
In a study on luteinizing hormone (LH) and FSH levels in women using oral contraception in 1993, the authors concluded, “FSH levels were rapidly suppressed from day 2 onward in all three groups,” and, “FSH levels are suppressed equally early and equally effective by all OCs studied.”32 (Hemrika et al., 1993)
Progestins also affect the action of FSH as described by Wright, 2020:
“It is believed that the hormonal contraceptive effect of progestin is inhibiting growth of active follicular tissue during the early follicular phase by reducing follicular sensitivity to FSH.”33 (Wright, Fayad, Selgrade, Olufsen, 2020)
LH and FSH deficiency reduces fertility as described here:
“Impairment of the production or action of gonadotropins causes relative or absolute LH and FSH deficiency that compromises gametogenesis and gonadal steroid production, thereby reducing fertility.”34 (Bosch et al., 2021)
And as previously noted, compromised follicle development leads to an inadequate corpus luteum.
The effects of oral contraceptives are stated plainly here:
“Another clinically significant topic concerning the corpus luteum is the use of oral contraceptives. Combined oral contraceptive pills contain 2 hormones, estrogen and progesterone, which suppress FSH and LH, thus inhibiting ovulation. Additionally, this suppression causes degeneration of the corpus luteum, resulting in a drop in progesterone levels, which inhibits normal implantation of the fertilized ova and placental attachment.”35 (Oliver, Pillarisetty, 2023)
Duncan previously explained how inadequate LH secretion leads to an inadequate corpus luteum. HC suppresses LH production which prevents the LH surge, or leads to blunted LH secretion.
“Since hormonal contraceptives similarly induce supraphysiologic levels of estrogens and progestins in cycles with escape ovulation, luteolysis may occur in these cycles as well. Blunted LH secretion is characteristic of cycles studied during the use of various hormonal contraceptives.”36 (Harrison, Buskmiller, Chireau, Ruppersberger, Yeung Jr, 2018)
Luteal phase deficiency is one of the main problems plaguing the IVF community, and it results from an inadequate corpus luteum.
And it’s the state a woman can find herself in if she conceives while using HC.
One study on the mechanism of action of Opill (a POP) reported that:
“…even the cycles [where women ovulated] classified as having a normal luteal phase may not have been optimal to support implantation or embryo development had conception occurred….Luteal phase deficiency is a condition in which endogenous progesterone is not sufficient to maintain a functional secretory endometrium or allow normal embryo implantation and growth. Although hard to diagnose in a clinical setting, it has been associated with subfertility and may contribute to the contraceptive effect of the POP.”37 (Glasier et al., 2022)
Why is Glasier et al. confessing about HC induced luteal phase deficiency working as an abortifacient?They’re not confessing. They’re bragging.
This is a feature for most birth control researchers, but it’s a bug for hedged contraceptionists.
Luteal phase support (exogenously administered progesterone) is the standard of care for women undergoing IVF to counter the deficiency. Women on HC who don’t know they’re pregnant won’t have that luxury.
In summary, pregnancy appears to be a finely-tuned process requiring embryo-endometrial synchrony throughout.
According to the research, hormonal contraception likely causes:
- endometrium desensitized to estrogen and progesterone, and
- decrease in LH and FSH production, leading to
- suboptimal follicle development, leading to
- inadequate corpus luteum in the event of ovulation, leading to
- underprepared endometrium, leading to
- embryo-endometrial desynchronization in the event of pregnancy, leading to
- worsened pregnancy outcomes
The theory that the corpus luteum will rescue a pregnancy doomed by CRIPLE seems little more than wishful thinking. The available evidence indicates this is unlikely.
Protestant hedged contraceptionists who believe pregnancies while on HC will be free from hazard should reconsider their position in light of available research.
But how often are women really getting pregnant on the pill?
How Often Might Abortions Occur on HC?
One paper uses data from research studies to make an estimation.
According to the authors, perfect use of a combined oral contraceptive could lead to a spontaneous abortion in 10 years using low ovulation rates (5%), or under 1 year using higher ovulations rates (69%).38 (Calcada, Alves, 2022)
Most other hormonal contraception classes, including progestin-only pills, indicated spontaneous abortion could occur in 1 year or less!
Read more about that paper here.
- McMahon, C., Hammarberg, K., Lensen, S., Wang, R., Mol, B. W., & Vollenhoven, B. J. N. (2024). What do women undergoing in vitro fertilization (IVF) understand about their chance of IVF success?. Human reproduction (Oxford, England), 39(1), 130–138. https://doi.org/10.1093/humrep/dead239 ↩︎
- Teh, W. T., McBain, J., & Rogers, P. (2016). What is the contribution of embryo-endometrial asynchrony to implantation failure?. Journal of assisted reproduction and genetics, 33(11), 1419–1430. https://doi.org/10.1007/s10815-016-0773-6 ↩︎
- Macklon, N. S., Geraedts, J. P., & Fauser, B. C. (2002). Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Human reproduction update, 8(4), 333–343. https://doi.org/10.1093/humupd/8.4.333 ↩︎
- Muter, J., Lynch, V. J., McCoy, R. C., & Brosens, J. J. (2023). Human embryo implantation. Development (Cambridge, England), 150(10), dev201507. https://doi.org/10.1242/dev.201507 ↩︎
- Devoto, L., Kohen, P., Muñoz, A., & Strauss, J. F., 3rd (2009). Human corpus luteum physiology and the luteal-phase dysfunction associated with ovarian stimulation. Reproductive biomedicine online, 18 Suppl 2, 19–24. https://doi.org/10.1016/s1472-6483(10)60444-0 ↩︎
- Devesa, J., & Caicedo, D. (2019). The Role of Growth Hormone on Ovarian Functioning and Ovarian Angiogenesis. Frontiers in endocrinology, 10, 450. https://doi.org/10.3389/fendo.2019.00450 ↩︎
- Abedel-Majed, M. A., Romereim, S. M., Davis, J. S., & Cupp, A. S. (2019). Perturbations in Lineage Specification of Granulosa and Theca Cells May Alter Corpus Luteum Formation and Function. Frontiers in endocrinology, 10, 832. https://doi.org/10.3389/fendo.2019.00832 ↩︎
- Gao, M., Cao, C., Zhang, X., Tang, F., Zhao, L., Luo, S., & Li, L. (2019). Abnormal expression of estrogen receptor is associated with thin endometrium. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology, 35(6), 544–547. https://doi.org/10.1080/09513590.2018.1554035 ↩︎
- DeWitt, N. A., Whirledge, S., & Kallen, A. N. (2020). Updates on molecular and environmental determinants of luteal progesterone production. Molecular and cellular endocrinology, 515, 110930. https://doi.org/10.1016/j.mce.2020.110930 ↩︎
- Tal R, Taylor HS. Endocrinology of Pregnancy. [Updated 2021 Mar 18]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278962/ ↩︎
- Bosch, E., Alviggi, C., Lispi, M., Conforti, A., Hanyaloglu, A. C., Chuderland, D., Simoni, M., Raine-Fenning, N., Crépieux, P., Kol, S., Rochira, V., D’Hooghe, T., & Humaidan, P. (2021). Reduced FSH and LH action: implications for medically assisted reproduction. Human reproduction (Oxford, England), 36(6), 1469–1480. https://doi.org/10.1093/humrep/deab065 ↩︎
- Bulletti, C., Bulletti, F. M., Sciorio, R., & Guido, M. (2022). Progesterone: The Key Factor of the Beginning of Life. International journal of molecular sciences, 23(22), 14138. https://doi.org/10.3390/ijms232214138 ↩︎
- Abuwala, N., & Tal, R. (2021). Endometrial stem cells: origin, biological function, and therapeutic applications for reproductive disorders. Current opinion in obstetrics & gynecology, 33(3), 232–240. https://doi.org/10.1097/GCO.0000000000000702 ↩︎
- Blanco-Breindel MF, Singh M, Kahn J. Endometrial Receptivity. [Updated 2023 Jun 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK587449/ ↩︎
- Hull, Louise, Robertson, Sarah, (2023, May 30), Trying for a baby? What you need to know about a vital part of your womb (and how to look after it, theconversation.com, https://theconversation.com/trying-for-a-baby-what-you-need-to-know-about-a-vital-part-of-your-womb-and-how-to-look-after-it-202854 ↩︎
- Benagiano, G., Mancuso, S., Guo, S. W., & Di Renzo, G. C. (2023). Events Leading to the Establishment of Pregnancy and Placental Formation: The Need to Fine-Tune the Nomenclature on Pregnancy and Gestation. International journal of molecular sciences, 24(20), 15420. https://doi.org/10.3390/ijms242015420 ↩︎
- Braun, A. S., Vomstein, K., Reiser, E., Tollinger, S., Kyvelidou, C., Feil, K., & Toth, B. (2023). NK and T Cell Subtypes in the Endometrium of Patients with Recurrent Pregnancy Loss and Recurrent Implantation Failure: Implications for Pregnancy Success. Journal of clinical medicine, 12(17), 5585. https://doi.org/10.3390/jcm12175585 ↩︎
- Yao, Y., Ye, Y., Chen, J., Zhang, M., Cai, X., & Zheng, C. (2024). Maternal-fetal immunity and recurrent spontaneous abortion. American journal of reproductive immunology (New York, N.Y. : 1989), 91(5), e13859. https://doi.org/10.1111/aji.13859 ↩︎
- Regidor P. A. (2018). Clinical relevance in present day hormonal contraception. Hormone molecular biology and clinical investigation, 37(1), 10.1515/hmbci-2018-0030. https://doi.org/10.1515/hmbci-2018-0030 ↩︎
- Murata, H., Tanaka, S., & Okada, H. (2021). Immune Tolerance of the Human Decidua. Journal of clinical medicine, 10(2), 351. https://doi.org/10.3390/jcm10020351 ↩︎
- Nagy, B., Szekeres-Barthó, J., Kovács, G. L., Sulyok, E., Farkas, B., Várnagy, Á., Vértes, V., Kovács, K., & Bódis, J. (2021). Key to Life: Physiological Role and Clinical Implications of Progesterone. International journal of molecular sciences, 22(20), 11039. https://doi.org/10.3390/ijms222011039 ↩︎
- Liao, Z., Liu, C., Cai, L., Shen, L., Sui, C., Zhang, H., & Qian, K. (2022). The Effect of Endometrial Thickness on Pregnancy, Maternal, and Perinatal Outcomes of Women in Fresh Cycles After IVF/ICSI: A Systematic Review and Meta-Analysis. Frontiers in endocrinology, 12, 814648. https://doi.org/10.3389/fendo.2021.814648 ↩︎
- Liji. (2022, December 29). How Does the Progestogen-only Pill Work? news-medical.net. https://www.news-medical.net/health/How-does-the-progestogen-only-pill-work.aspx. ↩︎
- Yland, J. J., Wesselink, A. K., Hernandez-Diaz, S., Huybrechts, K., Hatch, E. E., Wang, T. R., Savitz, D., Kuohung, W., Rothman, K. J., & Wise, L. A. (2023). Preconception contraceptive use and miscarriage: prospective cohort study. BMJ medicine, 2(1), e000569. https://doi.org/10.1136/bmjmed-2023-000569 ↩︎
- Homminga, I., Ter Meer, A. F., Groen, H., Cantineau, A. E. P., & Hoek, A. (2023). Thin endometrial lining: is it more prevalent in patients utilizing preimplantation genetic testing for monogenic disease (PGT-M) and related to prior hormonal contraceptive use?. Human reproduction (Oxford, England), 38(2), 237–246. https://doi.org/10.1093/humrep/deac258 ↩︎
- Wagner, L. H., Aurich, J., Melchert, M., Okada, C. T. C., Gautier, C., Kaps, M., Claaßen, S., & Aurich, C. (2023). Low progesterone concentration in early pregnancy is detrimental to conceptus development and pregnancy outcome in horses. Animal reproduction science, 257, 107334. https://doi.org/10.1016/j.anireprosci.2023.107334 ↩︎
- La Marca, A., Longo, M., Sighinolfi, G., Grisendi, V., Imbrogno, M. G., & Giulini, S. (2023). New insights into the role of LH in early ovarian follicular growth: a possible tool to optimize follicular recruitment. Reproductive biomedicine online, 47(6), 103369. https://doi.org/10.1016/j.rbmo.2023.103369La Marca, A., Longo, M., Sighinolfi, G., Grisendi, V., Imbrogno, M. G., & Giulini, S. (2023). New insights into the role of LH in early ovarian follicular growth: a possible tool to optimize follicular recruitment. Reproductive biomedicine online, 47(6), 103369. https://doi.org/10.1016/j.rbmo.2023.103369 ↩︎
- Nassaralla, C. L., Stanford, J. B., Daly, K. D., Schneider, M., Schliep, K. C., & Fehring, R. J. (2011). Characteristics of the menstrual cycle after discontinuation of oral contraceptives. Journal of women’s health (2002), 20(2), 169–177. https://doi.org/10.1089/jwh.2010.2001 ↩︎
- Gnoth, C., Godehardt, D., Godehardt, E., Frank-Herrmann, P., & Freundl, G. (2003). Time to pregnancy: results of the German prospective study and impact on the management of infertility. Human reproduction (Oxford, England), 18(9), 1959–1966. https://doi.org/10.1093/humrep/deg366 ↩︎
- Fields, C. Ryan. (2020). The Christian and Oral Contraceptives: An Investigation into Moral Permissibility. Dignitas 27, no. 1–4 (2020): 20–27. https://www.cbhd.org/dignitas-articles/the-christian-and-oral-contraceptives-an-investigation-into-moral-permissibility ↩︎
- Duncan W. C. (2021). The inadequate corpus luteum. Reproduction & fertility, 2(1), C1–C7. https://doi.org/10.1530/RAF-20-0044 ↩︎
- Hemrika, D. J., Slaats, E. H., Kennedy, J. C., de Vries Robles-Korsen, T. J., & Schoemaker, J. (1993). Pulsatile luteinizing hormone patterns in long term oral contraceptive users. The Journal of clinical endocrinology and metabolism, 77(2), 420–426. https://doi.org/10.1210/jcem.77.2.8345046 ↩︎
- Wright, A. A., Fayad, G. N., Selgrade, J. F., & Olufsen, M. S. (2020). Mechanistic model of hormonal contraception. PLoS computational biology, 16(6), e1007848. https://doi.org/10.1371/journal.pcbi.1007848 ↩︎
- Bosch, E., Alviggi, C., Lispi, M., Conforti, A., Hanyaloglu, A. C., Chuderland, D., Simoni, M., Raine-Fenning, N., Crépieux, P., Kol, S., Rochira, V., D’Hooghe, T., & Humaidan, P. (2021). Reduced FSH and LH action: implications for medically assisted reproduction. Human reproduction (Oxford, England), 36(6), 1469–1480. https://doi.org/10.1093/humrep/deab065 ↩︎
- Oliver R, Pillarisetty LS. Anatomy, Abdomen and Pelvis, Ovary Corpus Luteum. [Updated 2023 Jan 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539704/ ↩︎
- Harrison, D., Buskmiller, C., Chireau, M., Ruppersberger, L. A., & Yeung, P. P., Jr. (2018). Systematic Review of Ovarian Activity and Potential for Embryo Formation and Loss during the Use of Hormonal Contraception. The Linacre quarterly, 85(4), 453–469. https://doi.org/10.1177/0024363918815611 ↩︎
- Glasier, A., Edelman, A., Creinin, M. D., Han, L., Matulich, M. C., Brache, V., Westhoff, C. L., & Hemon, A. (2022). Mechanism of action of norgestrel 0.075 mg a progestogen-only pill. I. Effect on ovarian activity. Contraception, 112, 37–42. https://doi.org/10.1016/j.contraception.2022.03.022 ↩︎
- Calcada, M, Alves, A. (2022). Hormonal Contraceptives and Post-fertilization Effects. Issues in Law and Medicine, Spring 2022. https://issuesinlawandmedicine.com/wp-content/uploads/2023/09/Calcada.pdf ↩︎

